

Justin Hanft then introduced our speaker Robert Keem, Vice President of Operations for Athenex Pharma Solutions.
Rob earned a B.S. in Chemistry and a Master's in Analytical Chemistry from Buffalo State College, State University of New York. He began his career as a scientist focused on method development aand quality control testing at various biomedical companies. Rob has almost 30 years of management experience in operations, laboratory, and quality control/assurance departments. For at least 15 of those years, he has acquired knowledge from experiences in operational excellence, quality assurance, quality control and validation. Rob has managed departments and cross-functional teams of up to 100 reports (direct and indirect) in pharma, biotech and medical device companies (e.g. Thermofisher, Bristol Meyers Squibb, Lymphomed, and Athenex). Rob is proficient in the execution of compliance to cGMPs (CFR820/210/211), performing and hosting internal and external audits. Rob has been a team manager at Thermofisher Scientific (formerly Life Technologies) for over 10 years and was the Quality Lead for 6 sites which include Grand Island, NY; Bedford, MA; Logan, Utah; Inchinnan, Scotland; Auckland, New Zealand; and Oslo, Norway. His roles over those 10 years included method development and analysis in R&D, QC manager and Director/Sr. Manager of QA/QC. During his time as leader of operations, he was trained to six sigma methodologies including lean manufacturing, green belt, and root cause analysis. At Thermofisher as the Quality Lead, Rob developed supplier managment risk based programs including raw material qualification, vigorous metrics, robust management reviews and developed new capabilities within the organization and shared these best practices across sites and divisions of the company (e.g. stability program, improved test methodologies).
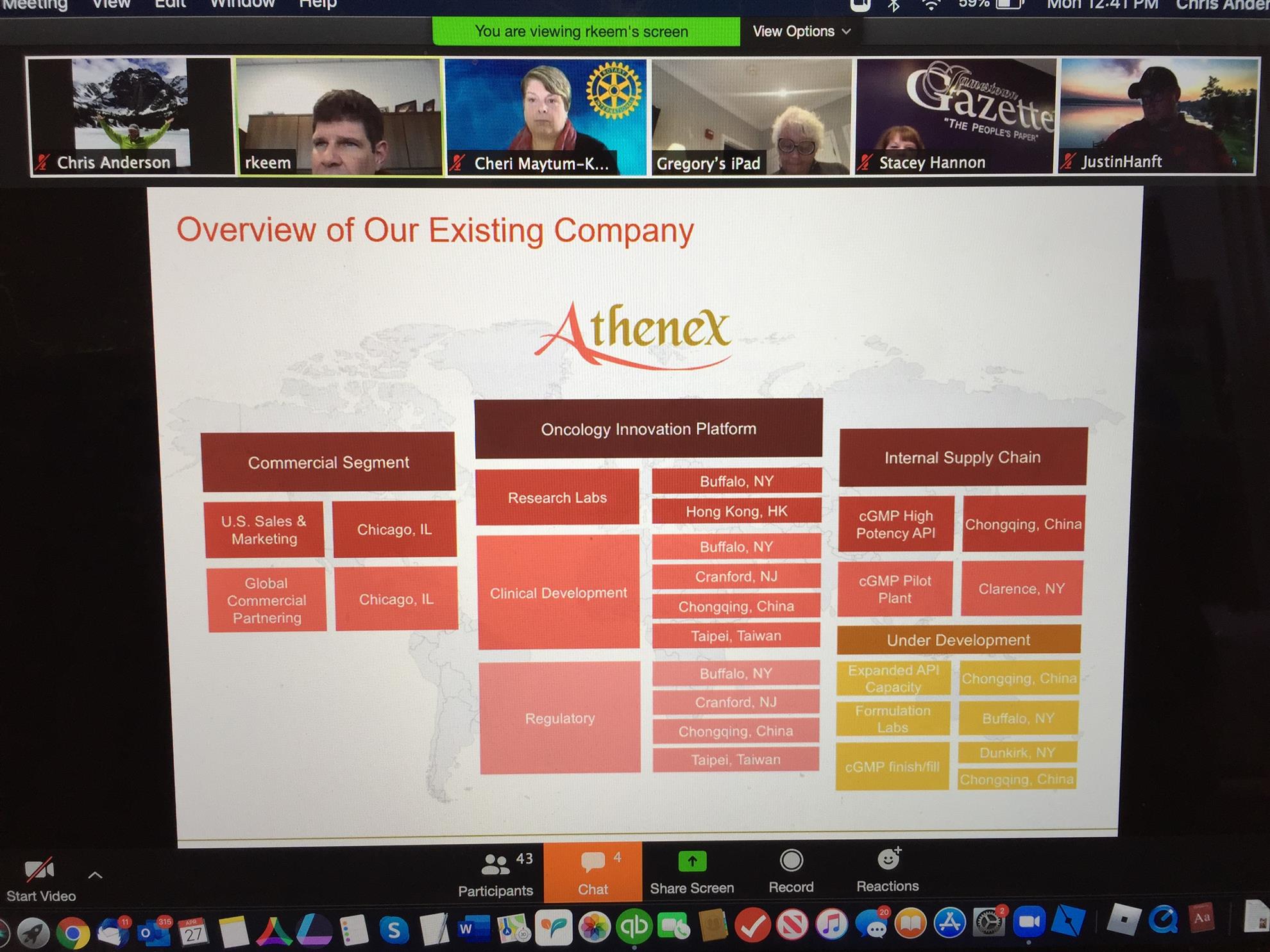
At his current company, Athenex, he has held roles as Vice President of Quality and currently as Vice President at Athenex Pharma Solutions (a division of Athenex). In his role as VP of Operations, he is leading the operational development of a topical proprietary ointment, the first drug that was initiated and to be commercialized in WNY in decades.
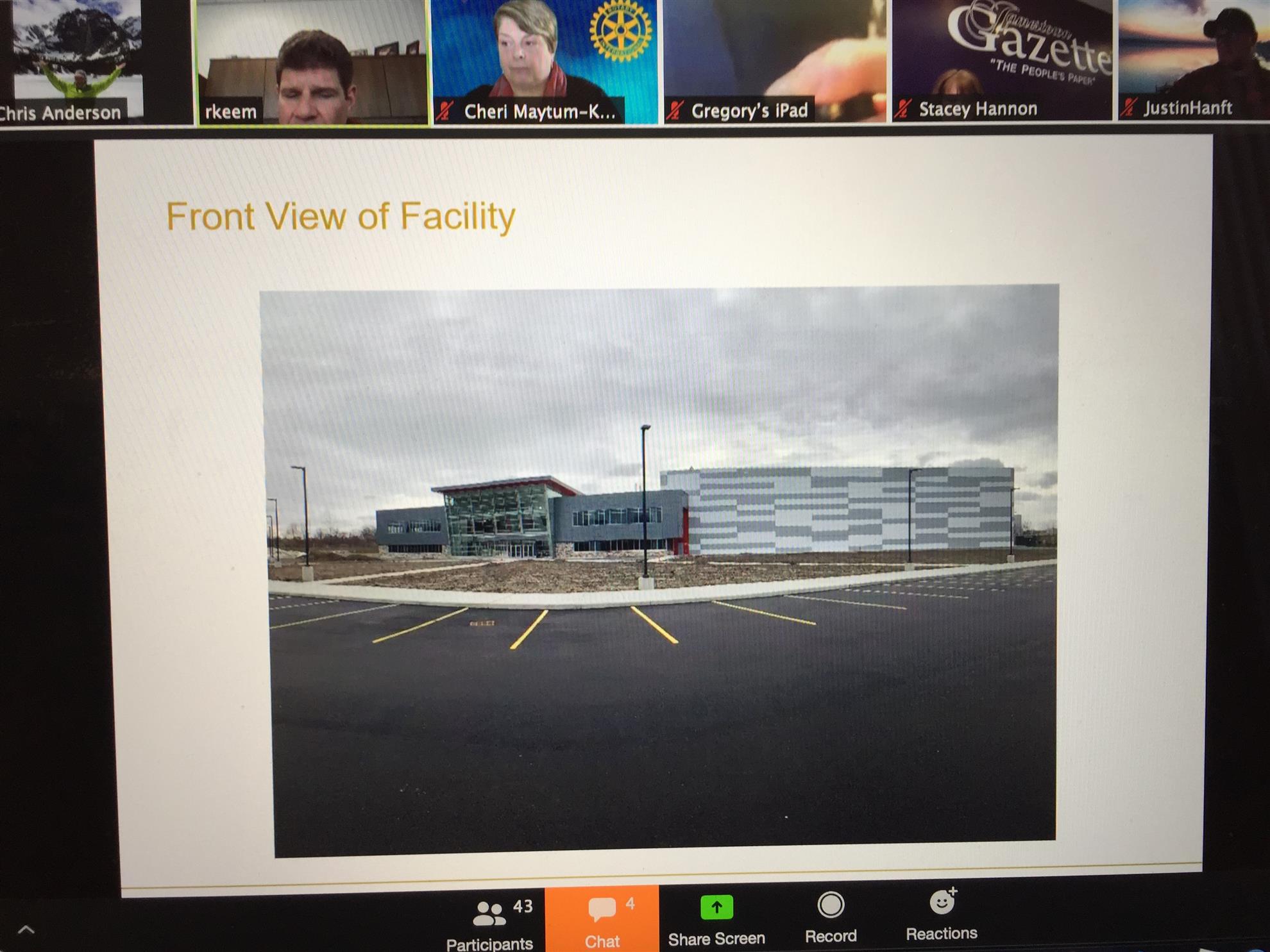
Construction of the Athenex Biotech plant began in the second quarter of 2017 and production is expected to begin in the late spring of 2020.
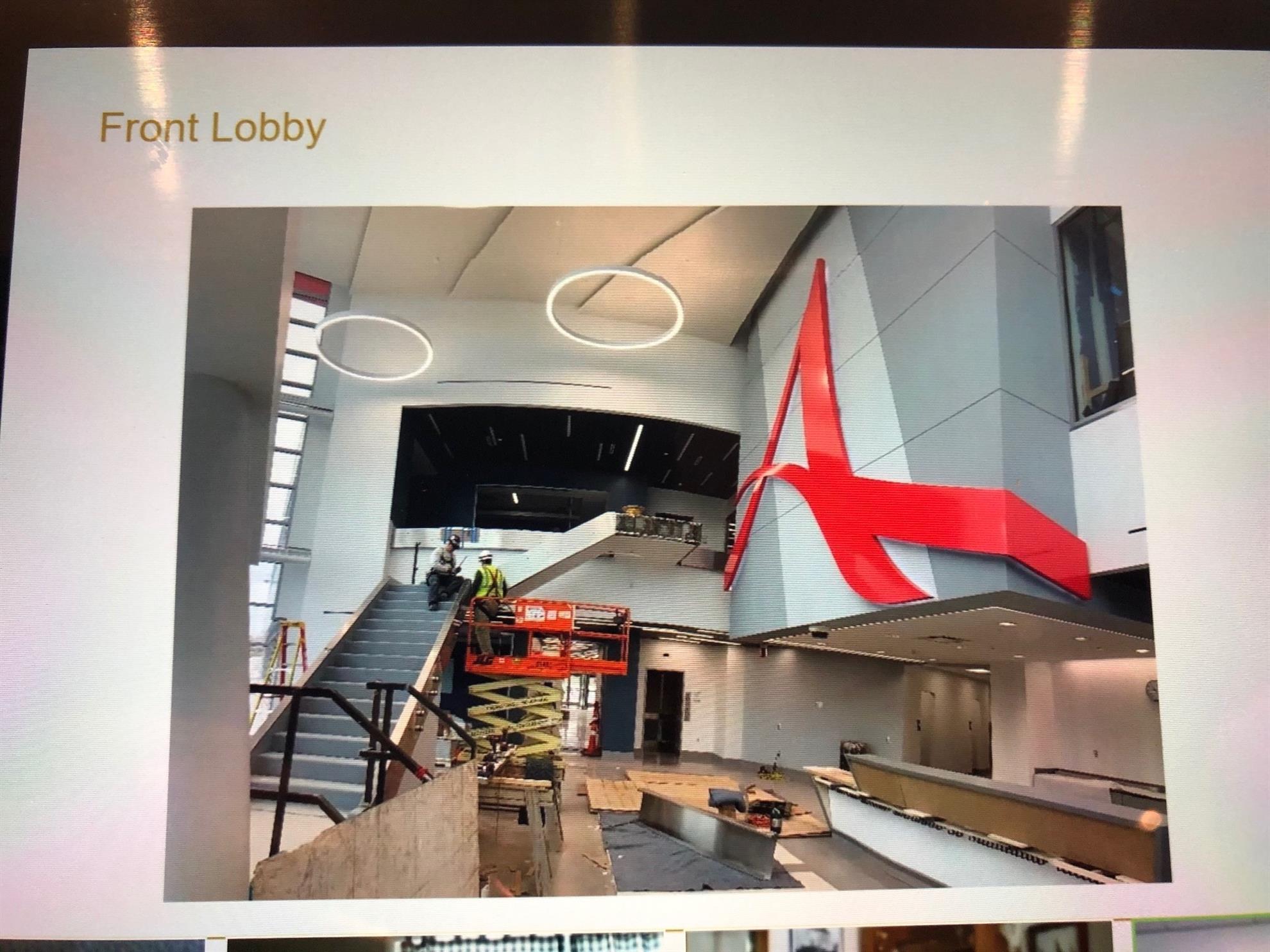
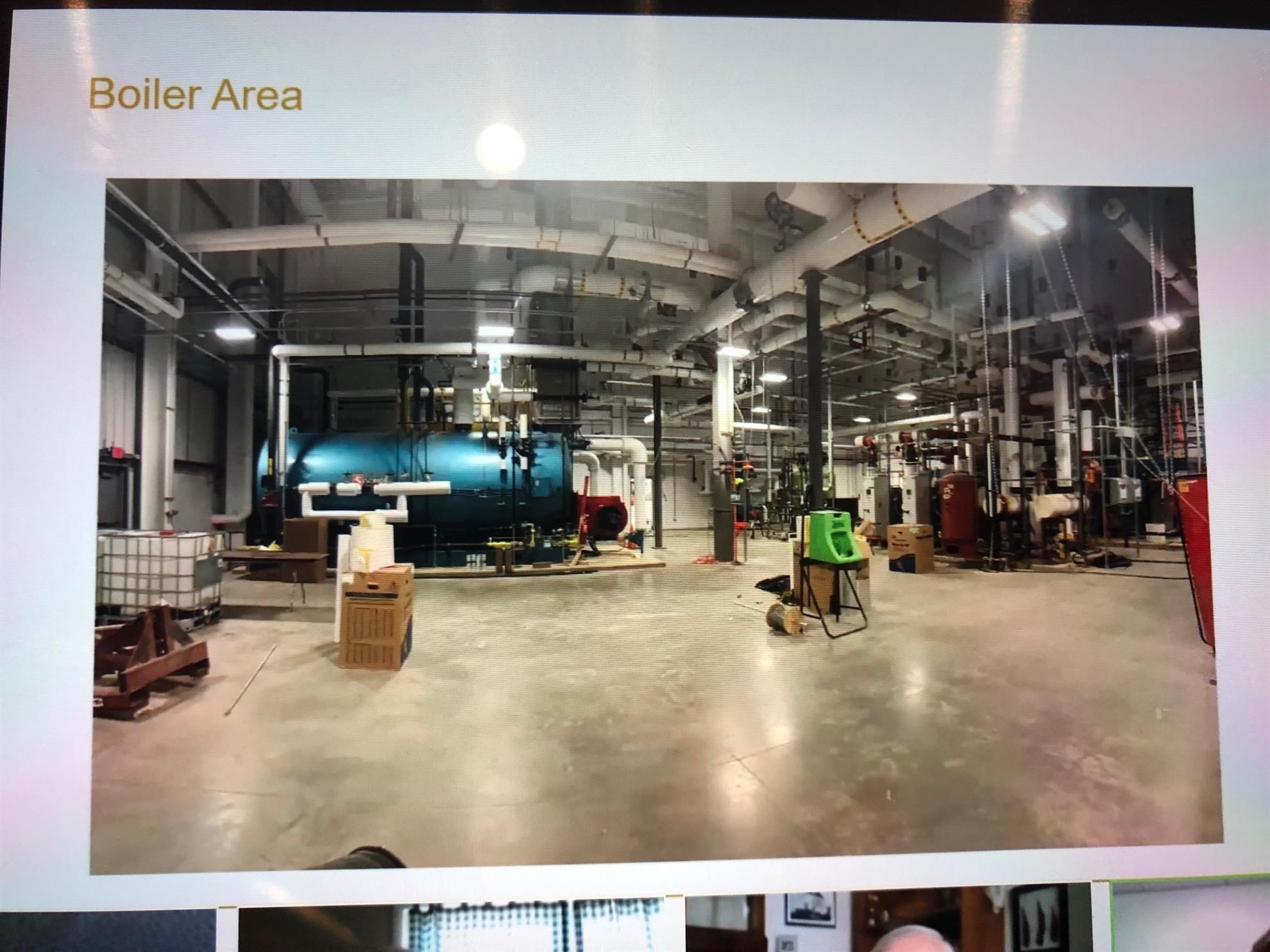
Athenex's new oncology manufacturing facility will occupy 400,000ft² area. In partnership with SUNY Polytechnic Institute, the high pharmacy oncology manufacturing facility in Dunkirk, New York, will manufacture sterile high-potency oncology drugs.
The plant will manufacture sterile, high-potency oncology drugs in a specialised and controlled, super-clean environment. Athenex is a speciality oncology drug manufacturing company based in the US, mainly engaged in the development and production of next-generation therapies for cancer diseases. Oncology pharmaceutical products, which are often listed in the FDA’s drug shortage list, will be manufactured for sales worldwide. Athenex is currently working on an oral breast cancer medication to replace the intravenous infusion presently in use.
As mentioned previously, they are also working on a small packet ointment to be utilized for actinic keratosis, a precursor to skin cancer.
Athenex is committed to produce innovative oncology drugs that deliver a life-changing impact on cancer patients. It is currently involved in the development of ten innovative and proprietary products.
The products are fascinating and so this editor does not misquote or misrepresent anything, she suggests that you Google Athenex so that you can read for about the products they are involved in developing and producing.
The new facility is expected to generate 900 jobs in areas such as high-tech manufacturing, product formulation, regulatory and pharmacovigilance. The oncology drug manufacturing facility is the first of its kind to be built in North America in the last 15 years.
Athenex will invest $1.52bn in the project, of which $200m will be invested by the New York state government, through the SUNY Polytechnic Institute.
The state government will contribute to the project as part of the Buffalo Billion Investment Development Plan, to support the regional economic development and create jobs in the western New York region.
The company’s headquarters in North America has a formulation product development center and a pilot plant, which mainly deals with the refinement of oncology drugs before the technology is further transferred to the new manufacturing facility in Dunkirk for large-scale production.
Rob indicated there will be MANY well-paying jobs available in many different categories - another reason for you to investigate their website. I only wish each of you who were not present could have heard and seen this presentation that really encouraged everyone in attendance as to the great future in store for Athenex, Dunkirk and the residents of this county. Rob is working hard with local schools, colleges and work force management firms to find and interest people to apply for these significant jobs.
Thank you to Rob Keem and all involved in Athenex for locating in Chautauqua County.